Charles R. Goulding and Ryan Donley take a look at the 3D printing impacts to come from a new medical acquisition.
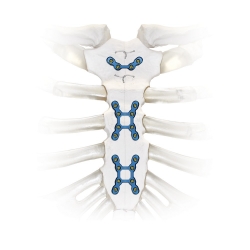
In November 2020 Zimmer Biomet, one of the international leaders in medical technology, announced that it will acquire A&E Medical. Zimmer (ZBH), a publicly traded company on the New York Stock Exchange (NYSE) worth approximately $8 billion, struck the deal to acquire A&E Medical for $250 million. A&E Medical is a company recognized for its medical equipment and device manufacturing. A&E Medical will contribute a full portfolio of sternal closure devices as well as temporary pacing wires and surgical punch products that will complement Zimmer Biomet’s growing profile of sternal closure products.
As stated by Bryan Hanson, President and CEO of Zimmer Biomet:
“A&E Medical’s high-growth business and innovative products are highly complementary to our current portfolio and will allow us to offer a comprehensive suite of sternal closure products, including rigid fixation, which has the potential to shift the standard of care and address a variety of unmet patient and surgical needs. This deal aligns with our active portfolio management strategy and the ongoing transformation of our business that will position Zimmer Biomet for long-term growth.”
Zimmer Biomet operates across numerous orthopedic implant fields which, in addition to sternal closure products, includes: knee, spine, foot, and ankle extremity segments. Furthermore, A&E Medical’s unique product line of sternal closure devices will not only complete Zimmer Biomet’s practice in this field but also the expand its 3D printing practices, as Zimmer Biomet has been steadily increasing its presence in the field of additive manufacturing (for various orthopedic implants).
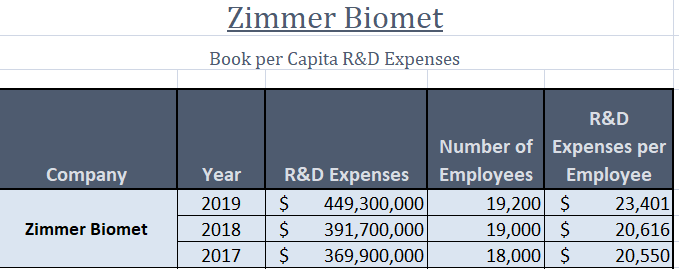
The merger of these orthopedic companies makes sense as both are dedicated to serving the same customer base and have similar business practices, including: sales, distribution, and financing initiatives. With a considerable investment in research and development, as shown below, Zimmer can gain a market advantage by overseeing R&D activities for A&E Medical’s products.
Examples of Zimmer’s 3D Printing Medical Practices
Zimmer Biomet had been an early adopter of additive manufacturing practices for medical technology development. Post-merger, additive manufacturing has ramped up significantly for the orthopedic giant. This led to the production of a wide-range of 3D printed medical devices and implants. The investment and use of SLM additive manufacturing/3D printing technology have paid dividends for Zimmer Biomet, particularly with the inception of the company’s own OsseoTi Porous Metal Technology, TrellOss Line, as well as several other 3D printed implants.
OsseoTi
OsseoTi Porous Metal Technology is a unique combination of Human CT data and 3D printing technology where a structure is built to directly mimic the architecture of the human cancellous bone. The fully integrated component contains solid and porous regions in order to maintain consistent strength to facilitate tissue in-growth and implant stability. The Ti6A14V alloy material used is a biocompatible and corrosion-resistant material that can create a very close attachment between the bone and the porous metal, which is important for the patient to essentially regain a natural range of motion.
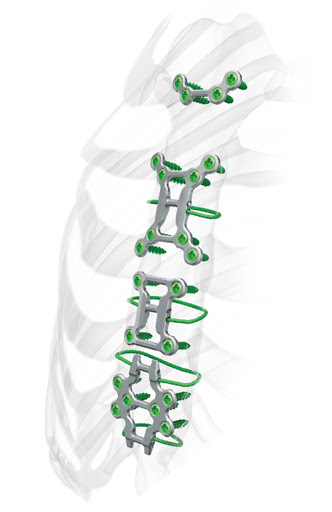
TrellOss Line
The TrellOss product line is comprised of interbody implants all with similar attributes. TrellOss products consist of 3D printed titanium interbody platforms with scaffold structures of 70% porosity and a surface
topography that can foster relevant cellular environments for bone adhesion and in-growth. The rigid teeth are a complex geometrical feat that was achieved with help from additive manufacturing technology. The result is the prevention of implant migration post-operation, which was problematic for many patients after surgery. The TrellOss-C is used as a cervical intervertebral fusion device and the Ti Interbody System is generally used in lumbar intervertebral fusion (LIF) surgeries for successful spinal fixation.
A&E Medical Potential in 3D Printing
After the surgery, there must be a surgical closure of the operated area. A&E Medical offers a unique product line of sternal closure devices that includes: sternal sutures, cable systems, and rigid fixation as well as a wide range of single-use complementary pacing wire and surgical punch products. Many of these devices have experimental provisions in 3D printing. This especially includes rigid fixation devices, which is a product Zimmer Biomet and A&E had developed separately prior to the acquisition. In fact, prior to the acquisition, A&E had its line of Tritium SCP Sternal Cable Plate and Zimmer Biomet had its SternaLock Blue. The two are very similar products that could stand to benefit from further experimentation, especially with 3D printing technology.
The complex nature of the surgery, the device geometry and patient anatomy make a “one-size-fits-all” type of device for sternotomy surgery nearly impossible. The combined expertise of the companies can greatly improve the precision of sternal device production practices. Further experimentation into alloy 3D printing for use with these medical devices could vastly improve sternal closure outcomes for patients.
3D printing and similar activities in the medical field may be eligible for R&D Tax Credits.
The Research & Development Tax Credit
Whether it’s used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Enacted in 1981, the now permanent Federal Research and Development (R&D) Tax Credit allows a credit that typically ranges from 4%-7% of eligible spending for new and improved products and processes. Qualified research must meet the following four criteria:
- Must be technological in nature
- Must be a component of the taxpayer’s business
- Must represent R&D in the experimental sense and generally includes all such costs related to the development or improvement of a product or process
- Must eliminate uncertainty through a process of experimentation that considers one or more alternatives
Eligible costs include US employee wages, cost of supplies consumed in the R&D process, cost of pre-production testing, US contract research expenses, and certain costs associated with developing a patent.
On December 18, 2015, President Obama signed the PATH Act, making the R&D Tax Credit permanent. Since 2016, the R&D credit has been used to offset Alternative Minimum Tax (AMT) for companies with revenue below $50MM and, startup businesses can obtain up to $250,000 per year in payroll tax cash rebates.
Conclusion
The acquisition of A&E Medical by Zimmer-Biomet presents a unique opportunity for further experimentation in the medical tools and technology field. The combination of A&E Medical’s capabilities in 3D printing of sternal implants and Zimmer Biomet’s expertise in 3D printing other medical devices and technologies, could lead to significant advancements in the fields of sternal implant surgeries, patient care, and several other surgery segments.