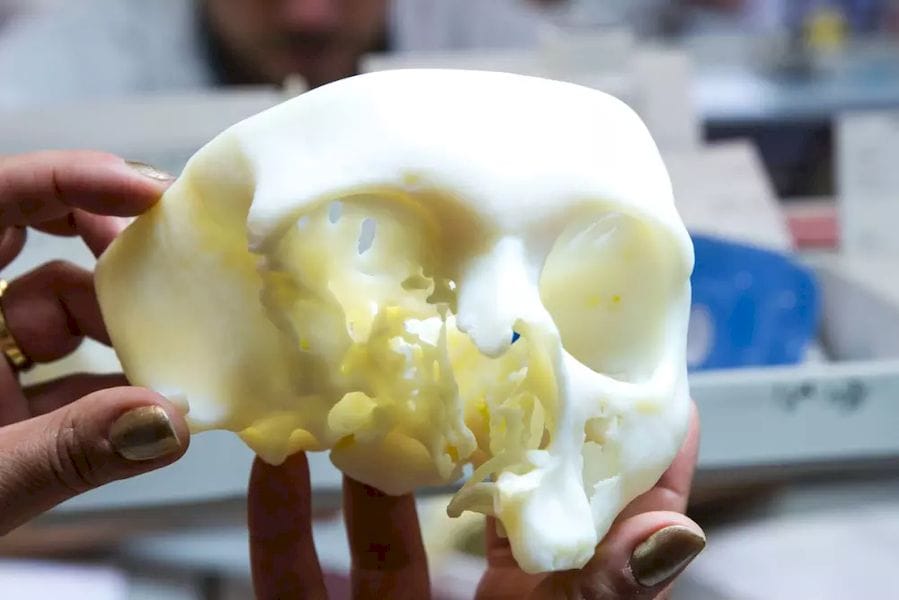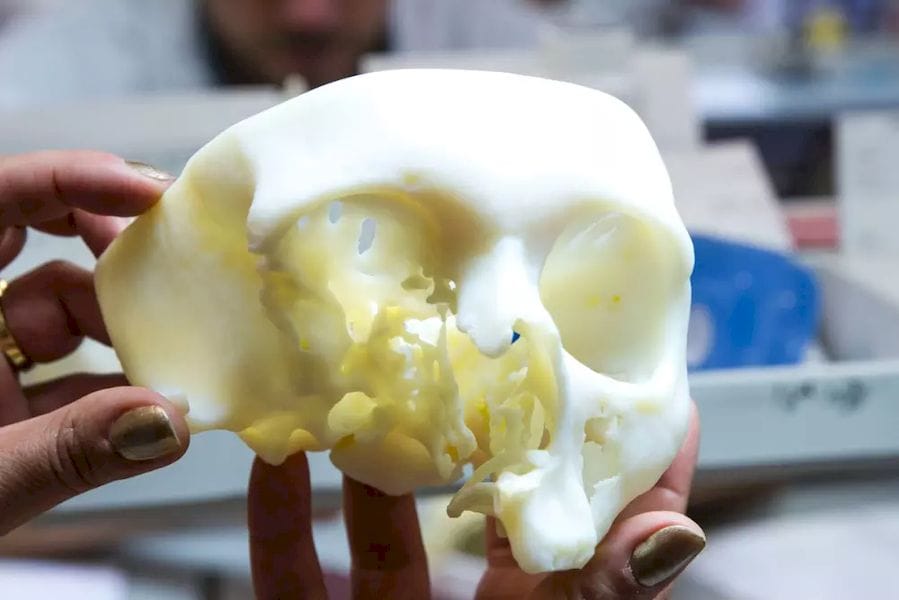
The U.S. Food and Drug Administration (FDA) has granted 3D printing service bureau and software developer Materialise clearance for the use of its Mimics inPrint software in the creation of anatomical models.
This is the first such clearance for a 3D printing software dedicated to 3D printing anatomical models for diagnostic purposes.
The medical space is among the first to experience a meaningful impact from 3D printing. This is in part due to the custom nature of the parts produced with the technology, as well as the unique geometries. As a result, over 37 different 3D-printed medical devices have received approval from the FDA.
The approval process is still piecemeal, with the FDA approving each 3D-printed component on a case-by-case basis. Now that Materialise has received FDA clearance for the use of Mimics inPrint, anatomical models can be used more readily in U.S. hospitals.
inPrint is a tool that makes it possible to convert patient medical image data, such as CT scans, to 3D models. This will allow the creation of patient-specific diagnostic models, which doctors can use for three-dimensional and tangible examination of scan data, potentially revealing affected areas that may have been missed through the use of traditional 2D medical images. Moreover, the models can be used to plan surgeries and communicate with doctors, medical students and patients about illness and treatment.
Read more at ENGINEERING.com