Could there be a path to truly safe, non-toxic 3D printer resin? New research indicates this could be the case.
3D printer resin is generally pretty toxic. That’s because it contains chemicals used to trigger polymerization called photoinitiators. When these molecules are exposed to certain frequencies of UV light, they bond together causing the material to become rigid.
This process creates “thermosets”, plastics that once solid remain that way. “Thermoplastics”, on the other hand, may be melted and reformed repeatedly, and you’ll find them used in FFF 3D printers. Resin 3D printers, however, use thermosets.
The problem is that these photoinitiators are toxic chemicals. If the human body is exposed to a sufficient quantity of them, it will develop a strong reaction that can manifest in several ways. It’s very possible to gradually accumulate toxins and then suddenly have a reaction erupt.
Some resins have lower amounts of toxic elements, and could be considered safer for use. But none are truly safe.
But now there is some new research that might point the way toward a truly safe resin.
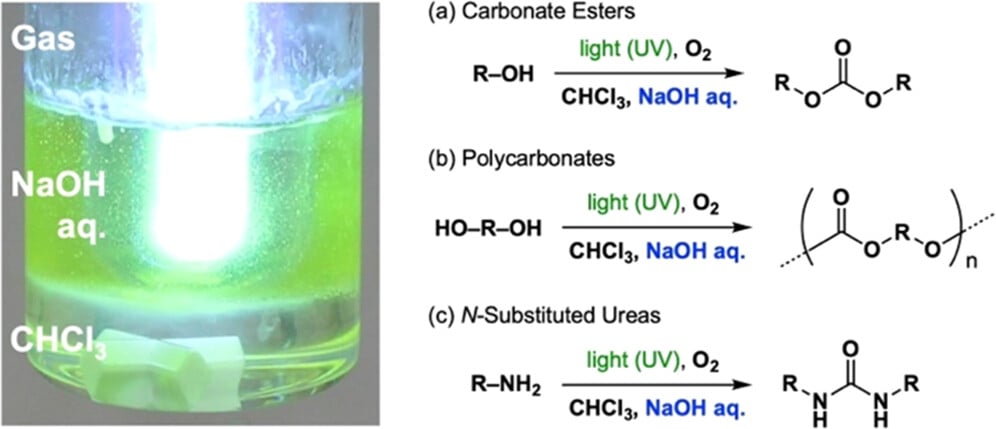
The researchers were interested in discovering a method to produce polycarbonate, a typical 3D print material most often used in FFF processes. Conventional production of “PC” involves the use of Phosgene, an extremely toxic chemical. How dangerous is this stuff? In World War I, approximately 77,000 soldiers were killed by Phosgene gas. It’s the same gas used to make PC.
The researchers report:
”Here, we report a novel photo-on-demand in situ phosgenation reaction that crosses three phases of a heterogeneous solution of chloroform (CHCl3) and aqueous NaOH containing an aryl alcohol or amine. This reaction system enables the safe, convenient, and inexpensive synthesis of carbonate esters, polycarbonates, and N-substituted ureas from aryl alcohols, aryl diols, and primary/secondary amines, respectively, on a practical scale and with good yield. The photochemical oxidation of CHCl3 to phosgene (COCl2) occurs upon irradiation with UV light from a low-pressure mercury lamp of both the gas and liquid phases of the reaction system under O2 bubbling of the vigorously stirred sample solution. The following reaction mechanisms are suggested: The aryl alcohol reacts in situ with the generated COCl2 at the interfaces of the organic/aqueous phases and aqueous/gas phases, in competition with the decomposition of COCl2 due to hydrolysis. Nucleophilicity and hydrophilicity are enhanced by the formation of aryl alkoxide ion through the reaction with NaOH, whereas the reaction of amine proceeds through neutralization of the generated HCl by the aqueous NaOH.”
Their process to produce PC involves illumination using UV light to produce in-situ phosgene that briefly exists to conduct the required chemical transformation.
Note that this is NOT a 3D printing process by any means; it’s simply a demonstration that this chemical reaction can occur.
The researchers explained:
“In this study, we report the first example of photo-on-demand in situ phosgenation reactions promoted by an inorganic base with heterogeneous mixtures of CHCl3 and aqueous alkaline solution containing an aryl alcohol or amine.”
I noticed that it involves use of UV light to trigger the process. But what if that UV light was selectively applied? Could PC be formed in certain regions and not others?
This sounds like it could potentially be the basis for a new style of 3D printing where a different form of resin and a selective UV light source could build PC objects.
Then, more surprisingly, they write:
“We show that this reaction is applicable to the synthesis of carbonate esters, polycarbonates, and N-substituted ureas.”
In other words, the basic approach they’ve devised could potentially be used to form other types of plastics.
While extremely early in the lifecycle, this discovery sounds quite promising and should absolutely be investigated as to whether it could be used in a more safe resin-based 3D printing process.
Via ACS

This uses chloroform, which is still highly toxic. It’s just that it’s liquid, so easier to handle than phosgene gas. And the NaOH is corrosive.