Charles R. Goulding and Arianna Coger examine the relationship between increased interest in ESG and additive manufacturing.
In Barron’s September 27th, 2021 issue, an article entitled “Medicine’s Golden Age” mentions how the period we are currently living in can be referred to as the Industrial Revolution for healthcare, the century of biology, Moore’s Law for medicine, or all three. Many investors are leaning towards impact investing and Environmental, Social, and Governance investing, which both focus on positively impacting society.
We’ve previously discussed the relationship between ESG investing and 3D printing for more efficient supply chains, inventory management, fuel usage, and recycling. Impact investors specifically seeking to fund businesses with goals that benefit society while generating financial returns should consider life science impact investing.
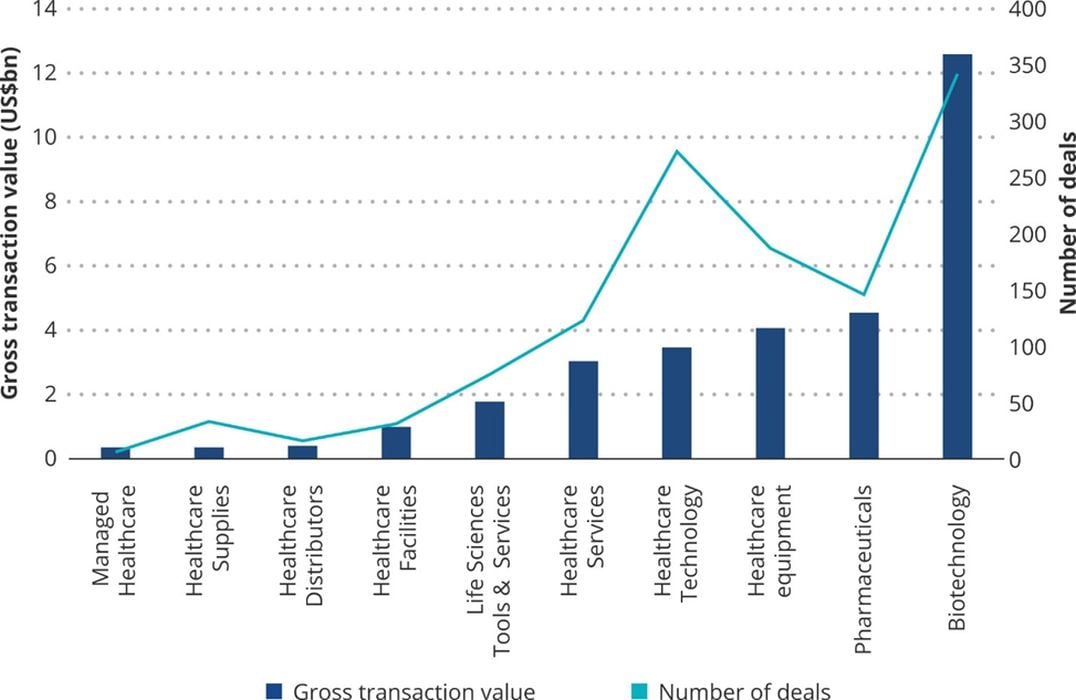
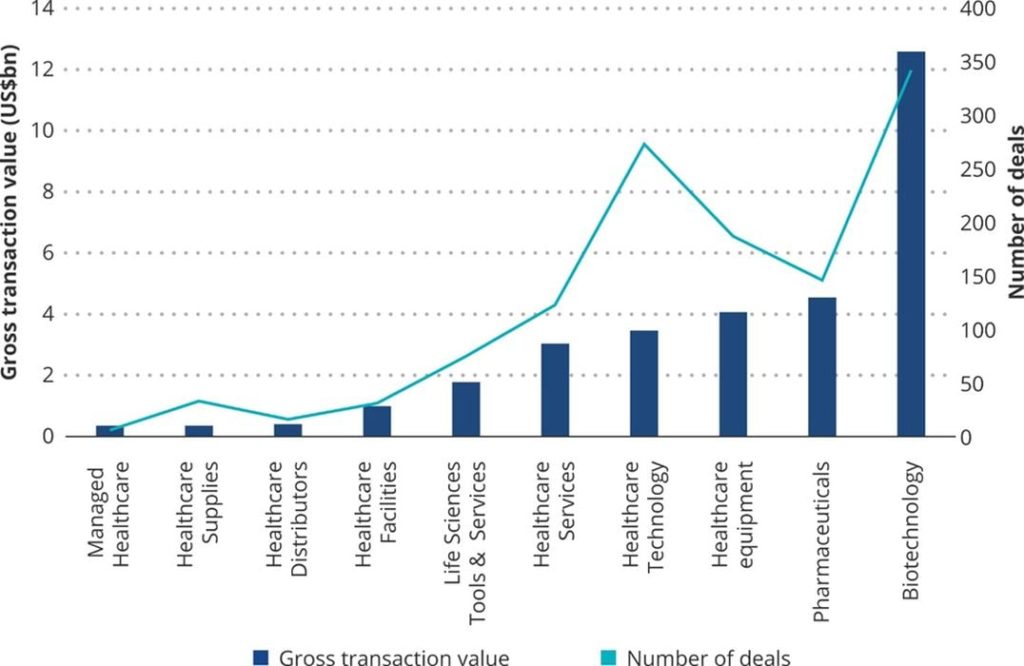
Pharmaceutical and biotechnology companies can be targets for socially conscious investing due to the common goal of improving health in society through new medicines, techniques, and technology. During 2020, the biotechnology market specifically saw a massive increase in interest from investors due to the COVID pandemic and the demand for solutions to recently exposed healthcare inefficiencies. This growth in healthcare interest has continued into 2021 and is expected to further expand in later years to improve health globally.
Although many impact investors may focus on the development of new drugs with technologies such as CRISPR or mRNA, there are also biotech companies that focus on the usage of 3D printing to develop novel treatments.
3D Printing in the Pharmaceutical and Biotech Industry
A key breakthrough event for the usage of 3D printing in the pharmaceutical industry occurred in 2015 with the FDA approval of Spritam, a medication used to treat epilepsy and manufactured with powder-bed inkjet printing. A 3D printing technology known as ZipDose was used to rapidly mass-produce the drug for distribution. Since then, multiple companies have worked towards achieving similar success with 3D printed drugs. However, refining and testing drug formulations is often a long, arduous, and expensive process. Due to the high costs of drug research and development, companies working in this area are often funded by investors to develop improvements for printing technology and to perform product testing.
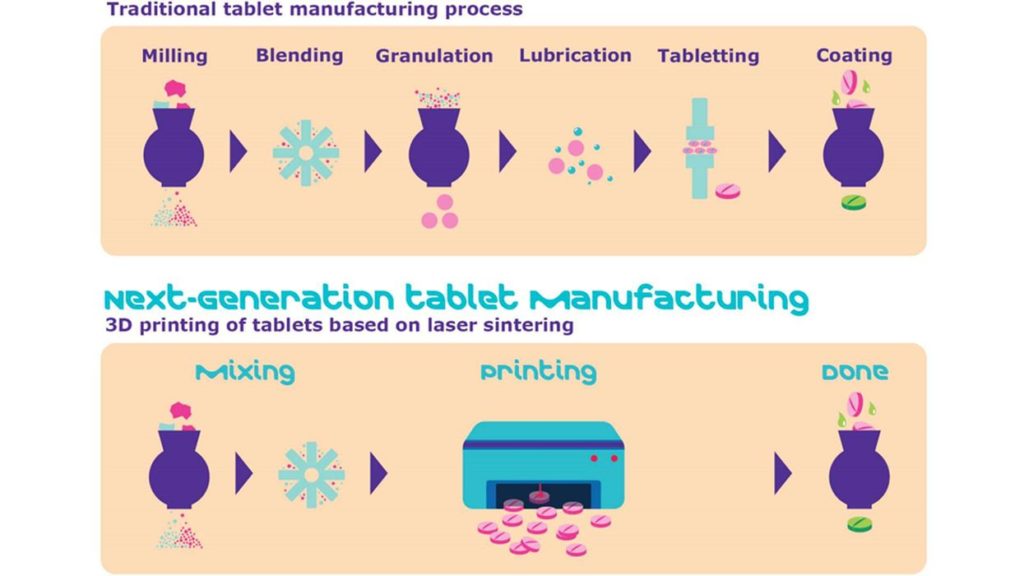
Although many companies have been performing research on 3D printing drugs, they often encounter difficulties while trying to translate from a laboratory setting to clinical practice. Streamlined drug delivery with 3D printing for more pharmaceutical products has been slow to come and so far, no other 3D printed drugs have gained FDA approval. Several factors have led to this delay in market approval and success. Some may include difficulties determining the appropriate excipients compatible with 3D printing, developing new printing software and instrumentation, and optimizing the mechanical properties of products. A more comprehensive understanding of the development process is needed to meet manufacturing safety and quality control standards while using 3D printing.
It has been suggested that the usage of machine learning (ML) can help accelerate the 3D printed drug development process. The amount of trial and error needed to develop a formulation compatible with 3D printing could be significantly reduced if ML were used to help narrow down viable excipients. ML techniques can also be utilized to improve quality control procedures by accurately simulating pharmaceutical workflow data.
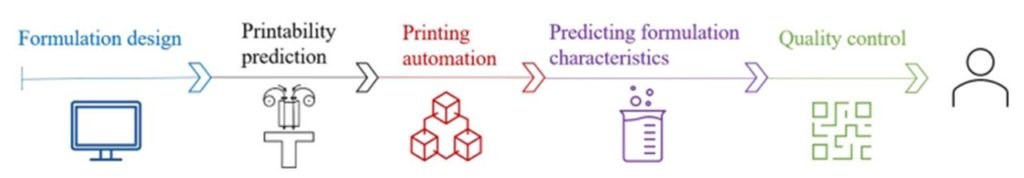
With or without ML, pharmaceutical and biotech research utilizing 3D printing will continue to increase and eventually a company is likely to successfully develop another FDA-approved 3D printed medication. The end goal for many researchers is to be able to rapidly develop personalized treatment for patients with 3D printing as the mode of production.
Companies participating in similar endeavors for advancing technology may be eligible for Research and Development Tax Credits.
The Research & Development Tax Credit
The now permanent Research and Development (R&D) Tax Credit is available for companies developing new or improved products, processes and/or software.
3D printing can help boost a company’s R&D Tax Credits. Wages for technical employees creating, testing, and revising 3D printed prototypes can be included as a percentage of eligible time spent for the R&D Tax Credit. Similarly, when used as a method of improving a process, time spent integrating 3D printing hardware and software counts as an eligible activity. Lastly, when used for modeling and preproduction, the costs of filaments consumed during the development process may also be recovered.
Whether it is used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Conclusion
Large investments of time and money are often needed to bring ideas to life in the healthcare industry. ESG and impact fund investors are often key components in funding new research and development efforts. 3D printing can be an important tool for developing new technologies and treatments that improve health and quality of life for all. Along with the development of new drugs, 3D printing is also often used to develop new medical devices and to develop human tissues for transplantation. As the technology continues to improve, we’re sure to see more 3D printing utilized by healthcare companies and eventually achieve the goal of personalized 3D printed medicine.