Charles Goulding Jr. considers the use of 3D printing in a different sort of pharmaceutical development.
Easy Does It
Oral Thin Films (OTFs) are drug strips that dissolve in the mouth. The elderly frequently prefer them, as do those with difficulty swallowing pills (37% of the population, according to a recent study).
Patient-friendliness is not the only key feature of OTFs. Perforations, as well as size and thickness adjustments, allow for highly customized dosing. These advantages allowed the OTF market to surpass $2bn in 2017. With an aging population, annual growth through 2026 may exceed 13%.
* Key players in the global oral thin films market are ZIM Laboratories Limited, Indivior plc, Aquestive Therapeutics, Inc., Sumitomo Dainippon Pharma Co., Ltd., IntelGenx Corp., Pfizer, Inc., Novartis AG, Allergan plc, NAL Pharma, and Solvay.
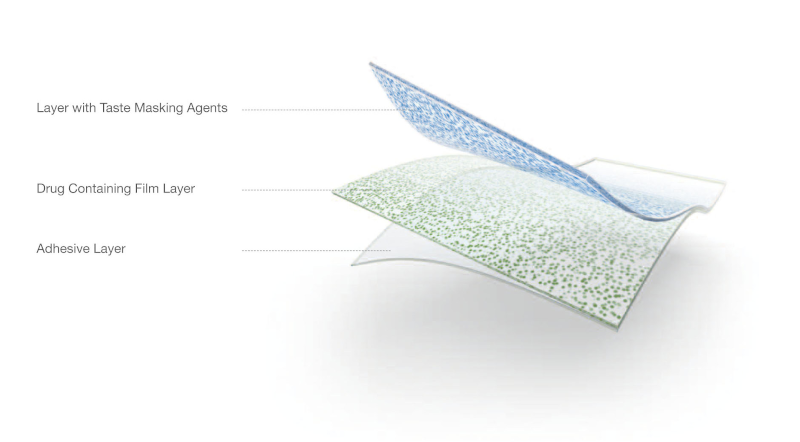
Leveling Up with 3D Printing
New research suggests 3D printing can both further distinguish OTFs for their customizability and also help overcome historical obstacles.
For customization, studies show that 3D printing enables subdivision of OTFs into layers of distinct active ingredients, as well as of excipients. This allows for a level of patient-specific personalization never before seen.
With respect to existing hurdles, OTFs have thus far been limited to water-soluble drugs. This is problematic since poorly soluble drugs comprise 40% of the overall market, and a whopping 90% of the drug development pipeline.
New research, however, suggests that 3D printing can indeed produce poorly soluble OTF polymers. If so, the future for this drug delivery system, already auspicious, may be even brighter.
More Innovation to Come
Other hurdles remain for OTFs. These include 1) size limitations that prevent higher-level doses and 2) packaging issues due to large surface areas that make OTFs sensitive to temperature change and mechanical stressors. However, studies suggest that a base film layer can be used to protect other layers, a solution that again underscores the intricate customization enabled by 3D printing.

The Research & Development Tax Credit
3DP firms innovating within OTFs and other drug delivery systems can avail themselves of the federal R&D tax credit to help subsidize their research efforts.
Enacted in 1981, the now permanent Federal Research and Development (R&D) Tax Credit allows a credit that typically ranges from 4%-7% of eligible spending for new and improved products and processes. Qualified research must meet the following four criteria:
- Must be technological in nature
- Must be a component of the taxpayer’s business
- Must represent R&D in the experimental sense and generally includes all such costs related to the development or improvement of a product or process
- Must eliminate uncertainty through a process of experimentation that considers one or more alternatives
Eligible costs include U.S. employee wages, cost of supplies consumed in the R&D process, cost of pre-production testing, U.S. contract research expenses, and certain costs associated with developing a patent.
On December 18, 2015, President Obama signed the PATH Act, making the R&D Tax Credit permanent. Beginning in 2016, the R&D credit can be used to offset Alternative Minimum Tax for companies with revenue below $50MM and for the first time, pre-profitable and pre-revenue startup businesses can obtain up to $250,000 per year in payroll tax cash rebates.
Conclusion
However complex OTFs become, they will remain easier to make than pills and capsules. That’s because the powderless, aqueous-based manufacturing process is continuous, in contrast to batch manufacturing with solvents. These advantages of customization and production, in addition to patient-friendliness, make OTFs a significant part of the drug-delivery ecosystem. If 3D printing can alter the OTF landscape as suggested by new research, the market for such drugs could grow even more precipitously.
