
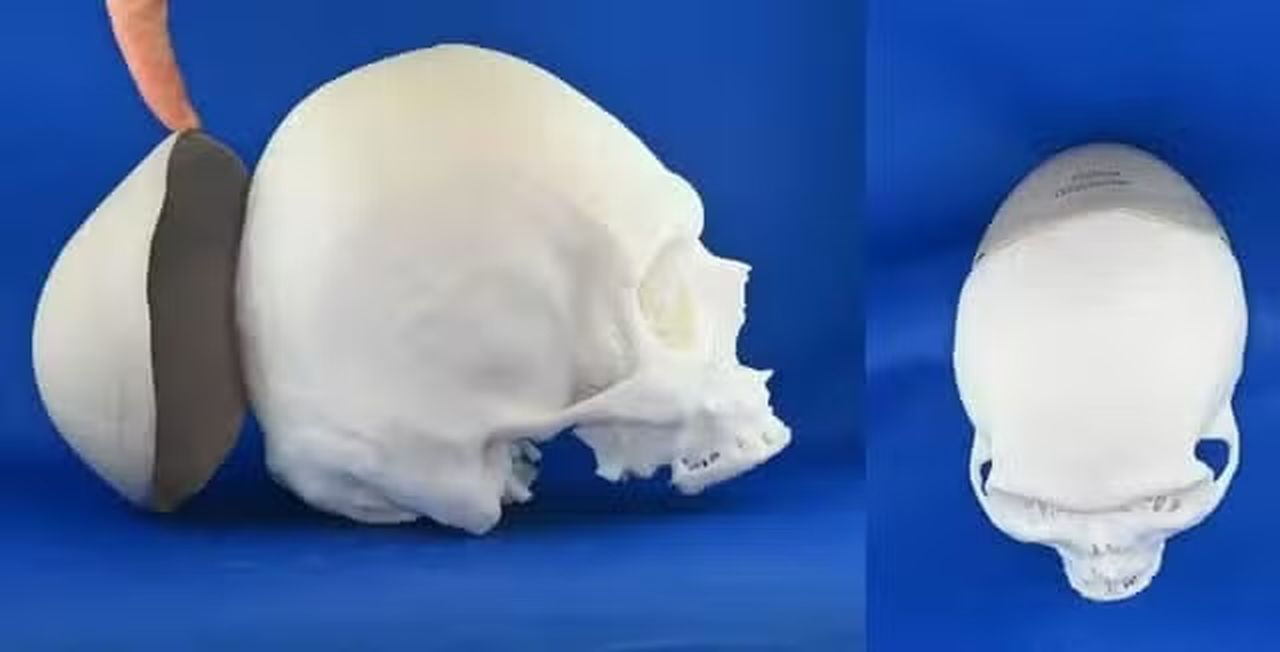
The FDA has approved the 3D printed, patient-specific VSP PEEK Cranial Implant solution.
The Food and Drug Administration (FDA) has provided 510(k) clearance for 3D Systems’ 3D printed, patient-specific cranial implant solution – VSP PEEK Cranial Implant. VSP PEEK Cranial Implant includes a complete FDA-cleared workflow comprising segmentation and 3D modeling software, the 3D Systems EXT 220 MED 3D printer, Evonik VESTAKEEP i4 3DF PEEK (polyetheretherketone), and a pre-defined production process.
By utilizing AM solutions, this technology can produce patient-specific cranial implants with up to 85% less material than similar implants produced by traditional machining, which can lead to significant cost savings for an expensive raw material like implantable PEEK.
Furthermore, the cleanroom-based architecture of the printer combined with simplified post-processing workflows makes it an ideal technology for producing patient-specific medical devices at the hospital site with faster turnaround while keeping the overall cost under control.
To date, this solution has been used to enable nearly 40 successful cranioplasties in Switzerland at University Hospital Basel, in Austria at Salzburg University Hospital, and in Israel at Tel-Aviv Sourasky Medical Center.
“3D printed PEEK cranial plates are an innovative solution that can improve patient care and expand the possibilities for precise, individualized neurosurgery,” said Dr. Johannes Pöppe, Senior Attending Surgeon at the Department of Neurosurgery in the University Hospital Salzburg. “The solution is revolutionizing the field. The combination of 3D Systems’ printing technology that is uniquely engineered for sterile environments along with the mechanical properties of PEEK are helping surgeons push boundaries. Within our hospital, we have already completed several successful surgeries using these technologies. I believe the potential for customized PEEK cranial plates is significant to integrate 3D printing into routine clinical practice.”
The VSP PEEK Cranial Implant is the first FDA-cleared, additively manufactured PEEK implant intended for cranioplasty procedures to restore defects in the skull. This implant-grade, high-performance polymer has a well-known clinical history in medical device applications due to its exceptional performance with mechanical properties closely mirroring human bone.
In addition, PEEK has excellent biocompatibility, resistance to bodily fluids, and stability in a wide range of temperatures. Furthermore, its inherent radiolucency ensures minimal interference in medical imaging – facilitating a clearer evaluation of the surgical site and implant integrity.
Read the rest of this story at VoxelMatters
