![Professor Tal Dvir, who led the research, stressed the importance of the heart's being made with a patient's own cells and biological materials in order to eliminate the risk of implant rejection. [Image: AFP via Straits Times]](https://fabbaloo.com/wp-content/uploads/2020/05/asvessel1504_img_5eb099422500b.jpg)
A cherry-sized bioprint may lead to 3D printed human hearts in a decade.
Bioprinting is a fascinating area of research. Much of it remains conceptual, and proofs-of-concept for bigger-picture ideas are in their earliest stages of testing. Still, any advance that shows some viability is worth exploring.
The implications of bioprinting are potentially massive. With aging populations around the world come the symptoms and ailments to go along with them: disease, deterioration. Organ transplants are a life-saving treatment option, but due to the nature of organ donation there is always a much shorter supply than there is demand. When a matching donor is found, a life-long regimen of anti-rejection therapies remain ahead, assuming the transplant itself is successful.
Heart disease in particular is in focus, as it is high among the leading causes of death: second in Israel to cancer, first in the US.
But what if, say the starry-eyed scientists, we could make organs for those who need them? Not just organs-on-demand, but matching organs-on-demand, made just for the people who need them?
Enter bioprinting. While full-organ transplants remain years away, timelines may be getting more realistic as work continues in labs around the world.
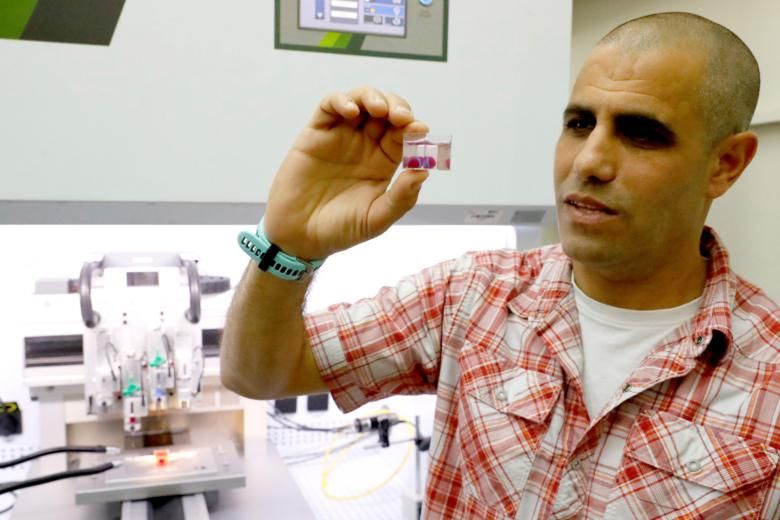
Today, scientists from Tel Aviv University have announced their latest lab success: a 3D printed heart, sized for a rabbit, with full internal structuring.
“This is the first time anyone, anywhere, has successfully engineered and printed an entire heart replete with cells, blood vessels, ventricles and chambers,” said research leader Professor Tal Dvir.
Dvir and his team took what their resulting paper calls “a simple approach” to 3D print cardiac patches. Based on a biopsy of a patient’s own heart, a patient-specific bioink is created with sugars and proteins, with cells reprogrammed as necessary to function as cardiac or endothelial cells.
That “simple approach” is indeed rather involved.
![Concept schematic. An omentum tissue is extracted from the patient and while the cells are separated from the matrix, the latter is processed into a personalized thermoresponsive hydrogel. The cells are reprogrammed to become pluripotent and are then differentiated to cardiomyocytes and endothelial cells, followed by encapsulation within the hydrogel to generate the bioinks used for printing. The bioinks are then printed to engineer vascularized patches and complex cellularized structures. The resulting autologous engineered tissue can be transplanted back into the patient, to repair or replace injured/diseased organs with low risk of rejection. [Image: Tel Aviv University]](https://fabbaloo.com/wp-content/uploads/2020/05/advs1070-fig-0001-m1_img_5eb099428d518.jpg)
Because of the use of an individual’s own tissue, the resulting structures should be fully compatible upon implantation.
The scientists suggest that long-term studies are in order for next steps: first in animals then, eventually, in human subjects.
Of course, that path is not necessarily so linear as moving straight into the next lab. Dvir and his team note a few of many lingering challenges in their paper’s conclusion:
“Although 3D printing is considered a promising approach for engineering whole organs, several challenges still remain. These include efficient expansion of iPSCs to obtain the high cell number required for engineering a large, functioning organ. Additionally, new bioengineering approaches are needed to provide long‐term cultivation of the organs and efficient mass transfer, while supplying biochemical and physical cues for maturation. The printed blood vessel network demonstrated in this study is still limited. To address this challenge, strategies to image the entire blood vessels of the heart and to incorporate them in the blueprint of the organ are required. Finally, advanced technologies to precisely print these small‐diameter blood vessels within thick structures should be developed.”
“iPSCs” are induced pluripotent stem cells, which form the basis of the new cells.
![Printing thick vascularized tissues. a) A top view of a lumen entrance (CD31; green) in a thick cardiac tissue (actinin; pink). b) A model of a tripod blood vessel within a thick engineered cardiac tissue (coordinates in mm), and c) the corresponding lumens in each indicated section of the printed structure. d) Tissue perfusion visualized from dual viewpoints. e–k) A printed small‐scaled, cellularized, human heart. e) The human heart CAD model. f,g) A printed heart within a support bath. h) After extraction, the left and right ventricles were injected with red and blue dyes, respectively, in order to demonstrate hollow chambers and the septum in‐between them. i) 3D confocal image of the printed heart (CMs in pink, ECs in orange). j,k) Cross‐sections of the heart immunostained against sarcomeric actinin (green). Scale bars: (a,c,h, i,j) = 1 mm, (g) = 0.5 cm, (k) = 50 µm. [Image: Tel Aviv University]](https://fabbaloo.com/wp-content/uploads/2020/05/advs1070-fig-0006-m1_img_5eb0994310caf.jpg)
Despite the existing limitations, the team remain confident in potential applicability with what is an ambitious time frame:
“Maybe, in 10 years, there will be organ printers in the finest hospitals around the world, and these procedures will be conducted routinely,” Dvir said.
While perhaps not “routine” in the next decade, the new research is another step forward for bioprinting. Research into cardiac applications will continue to build upon earlier studies, and perhaps in the next generation we will indeed see made-to-order hearts.
Via Jerusalem Post and Advanced Science










FELIXprinters has released a new bioprinter, the FELIX BIOprinter, which is quite a change for the long-time 3D printer manufacturer.