Charles R. Goulding and Mwansa Chabala look at how four medtech companies leveraged 3D printing to recover after the pandemic.
During the deferred surgery Covid period we suggested that the 3D printing industry take the time to improve their technology and do more training. An April 13th Wall Street Journal Article (WSJ) begins by saying “Medical Device Makers stocks are basking in good times”. The WSJ article focuses on 4 leading medical device makers namely Boston Scientific, Edwards Life Sciences, Stryker, and Medtronic that are enjoying a post-Covid resurgence.
Boston Scientific Corporation
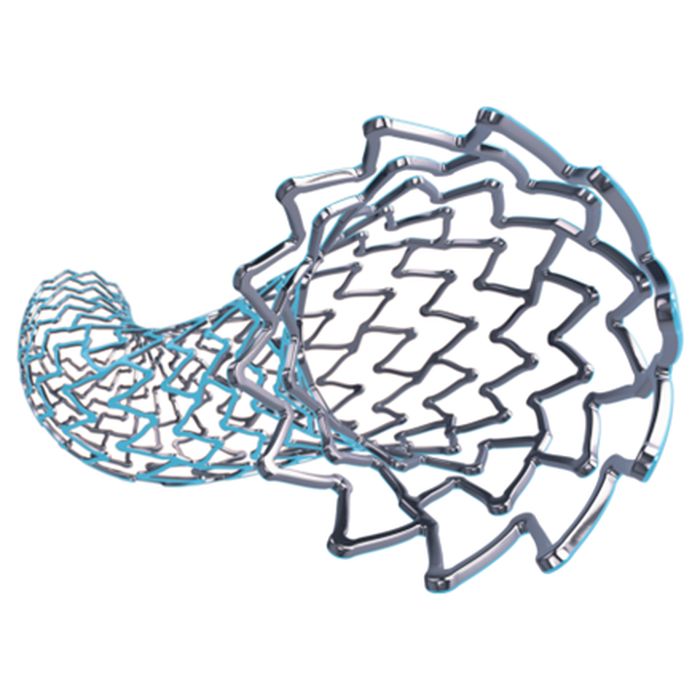
Boston Scientific is a global leader in medical technology, renowned for its innovative devices like the Taxus Stent used to treat coronary artery disease. With 51,000 employees worldwide and generating US$12.68B in revenue in 2022, they specialize in a diverse range of medical devices such as pacemakers, defibrillators, catheters, and other implantable devices used to treat cardiovascular disease, chronic pain, urological and pelvic health conditions, gastrointestinal disorders, and more. Previously we have outlined how they tackle medical challenges using 3D printing.
Edwards Lifesciences

Edwards Lifesciences is a leading global innovator in medical technology, focusing on structural heart disease, critical care, and surgical monitoring. With over 14,000 employees and sales of US$5.38B in 2022, the company specializes in cardiovascular technologies, including transcatheter and surgical heart valves, critical care monitoring systems, and other products. Edwards Lifesciences’ latest breakthrough is the PASCAL Precision system for treating degenerative mitral regurgitation. Their ongoing research and development efforts demonstrate their commitment to improving patient outcomes, and their patient-centric solutions have established them as a global leader in cardiovascular medicine.
Stryker Corporation
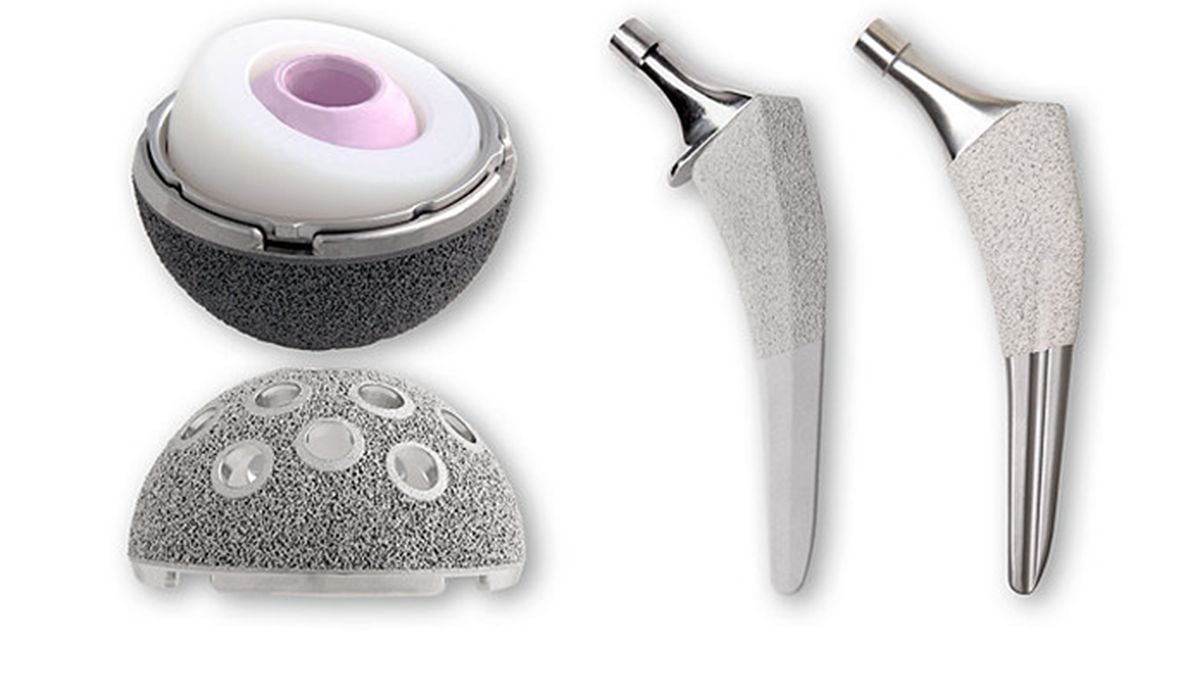
Stryker, a leading global medical technology corporation based in Michigan, has over 51,000 employees and generated US$18.4B in sales in 2022. They specialize in several medical specialties, including Medical and Surgical, Neurotechnology, Orthopaedics, and Spine, and invest significantly in research and development with a US$1.45B budget in 2022 alone. One example of their innovation is the Trident II Tritanium technology, designed to promote long-term biologic fixation in orthopedic procedures.
Stryker is also dedicated to sustainability and social responsibility, implementing various initiatives to reduce their environmental impact and improve access to healthcare globally. Some of its 3D ventures are explored in our earlier article.
Medtronic PLC

Medtronic, one of the largest medical device companies worldwide, employs over 90,000 people and invests US$2.7B in R&D. Their innovative healthcare technologies and therapies serve over 72 million people annually in 150 countries, with products spanning pacemakers, insulin pumps, heart valves, surgical tools, and more. We outlined Medtronic’s acquisition of medical technology through 3D printing in our 2020 article. The company generated US$31.69B USD in revenue in 2022, reflecting its position as a leader in the industry. Medtronic is also committed to sustainability, social responsibility, and improving access to healthcare globally.
The Research & Development Tax Credit
The now permanent Research and Development (R&D) Tax Credit is available for companies developing new or improved products, processes and/or software.
3D printing can help boost a company’s R&D Tax Credits. Wages for technical employees creating, testing, and revising 3D printed prototypes can be included as a percentage of eligible time spent for the R&D Tax Credit. Similarly, when used as a method of improving a process, time spent integrating 3D printing hardware and software counts as an eligible activity. Lastly, when used for modeling and preproduction, the costs of filaments consumed during the development process may also be recovered.
Whether it is used for creating and testing prototypes or for final production, 3D printing is a great indicator that R&D Credit eligible activities are taking place. Companies implementing this technology at any point should consider taking advantage of R&D Tax Credits.
Conclusion
Despite the disruption caused by the COVID-19 pandemic within the industry, companies are adapting to new challenges and the changing demands. With the integration of cutting-edge 3D printing technology, medical device makers are working to go back to normal and continue their commitment to developing innovative solutions that improve patient outcomes and quality of life. The healthcare industry will continue to rely on these companies to drive progress and advance the field of medical technology.
